What is a Certificate of Analysis (COA)?
What is a Certificate of Analysis (COA)?
A Certificate of Analysis (COA) is an official lab report that verifies the contents, potency, and safety of general raw materials or cannabis products. It is issued by a licensed third-party laboratory after testing and typically includes:
-
Potency Results – THC, CBD, and other cannabinoids
-
Terpene Profile – flavor and aroma compounds
-
Contaminant Screening – pesticides, heavy metals, mold, microbials
-
Residual Solvents (for extracts and concentrates)
-
Lot / Batch Identification – links the lab results to specific production batches
Purpose:
-
Ensures compliance with state regulatory bodies (e.g., METRC integration in CO, MO, etc.)
-
Provides transparency and safety assurance to consumers and buyers
-
Serves as a chain of custody document, proving that products meet legal and quality standards
Where is the COA stored in Figgro?
In the Figgro ERP (Seed-to-Sale) system, COAs are treated as compliance and quality documents tied to inventory and product records. Typically, COAs are:
-
Uploaded into the Quality & Compliance Module
-
Stored against specific lots, batches, or production runs.
-
Accessible when creating manifests or sales orders.
-
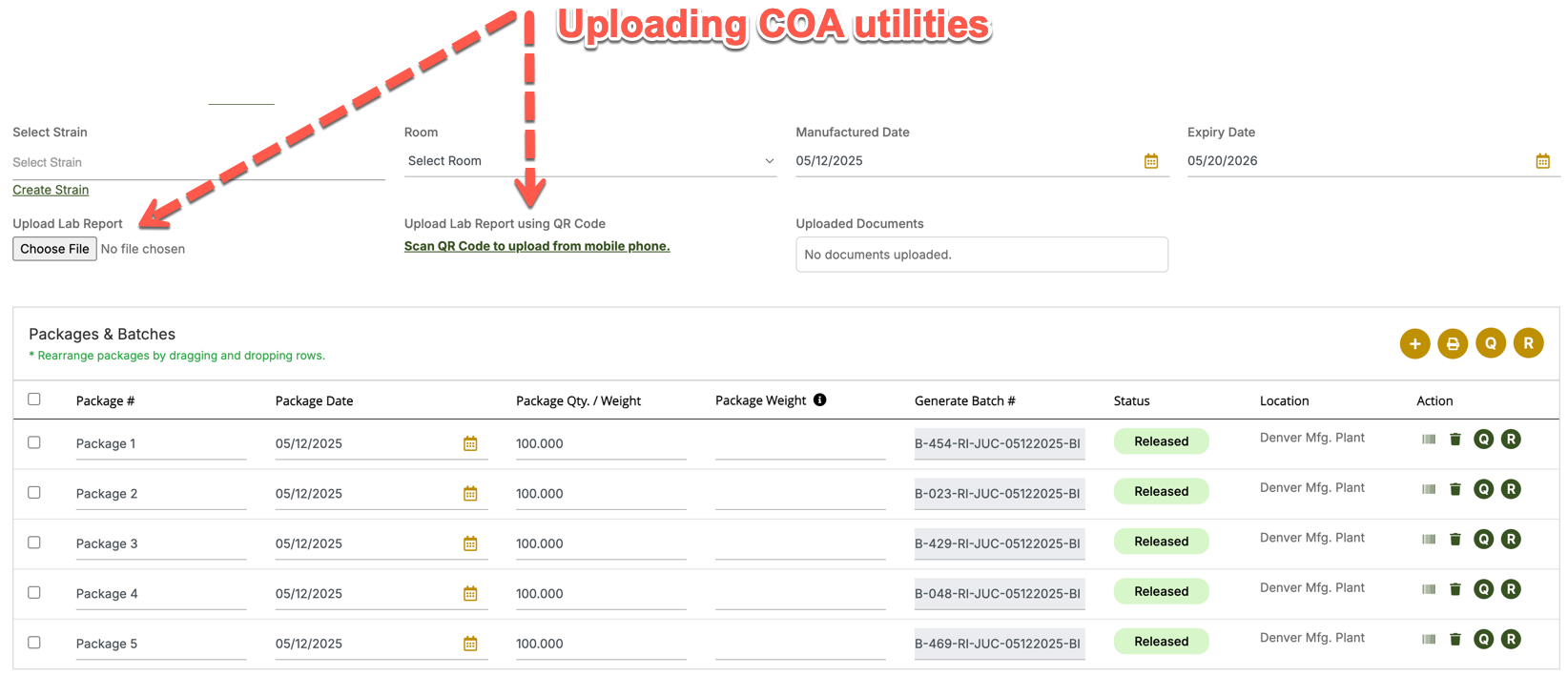
-
Linked at the Product / Package Level
-
Each finished good, bulk ingredient, or raw material has an associated COA.
-
If a product is transferred, the COA automatically follows the manifest.
-
-
Attached to Sales & Distribution Documents
-
Buyers ( distributors, manufacturers) can download/view COAs directly from Figgro.
-
Helps streamline audits and customer trust.
-
-
Archived in the Document Repository
-
COAs remain in secure cloud storage for audit trails, with version history and expiration dates and can be found under the Chain of Custody Module---> Manage Lab Reports sub module.
-
Ensures alignment with GMP/HACCP compliance by maintaining chain-of-custody traceability.
-
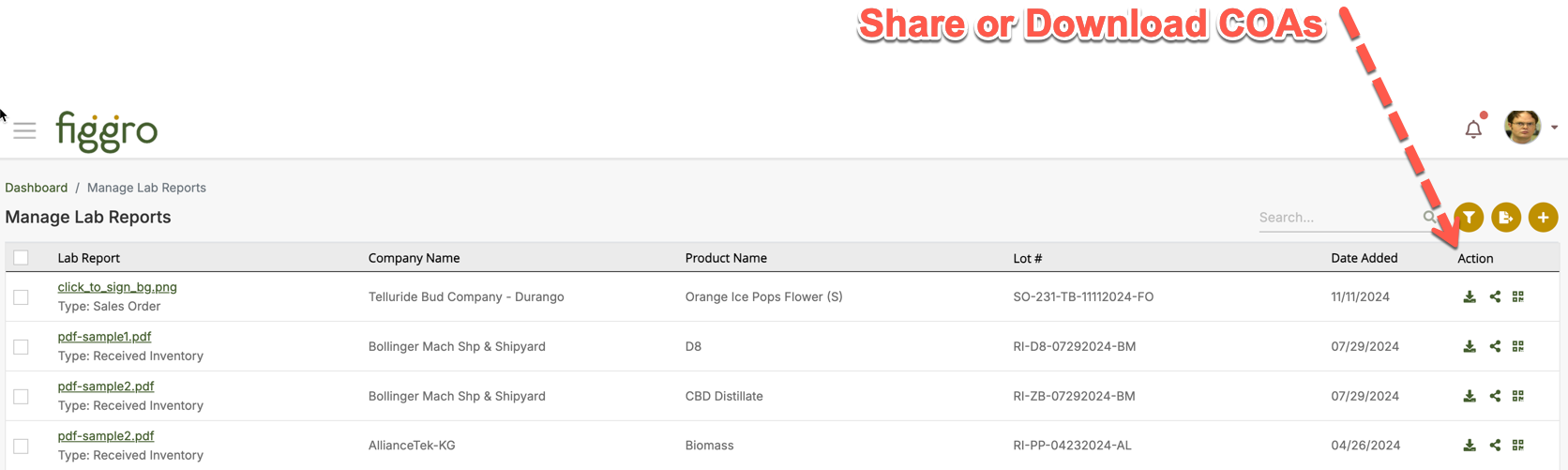
In short: A COA in Figgro lives in the Inventory / Compliance layer, directly tied to batches and packages. This ensures it is always available for regulators, internal QA teams, and buyers during manifests, transfers, or audits.
![figgro_logo_TM_Green-1.png]](https://university.figgro.com/hs-fs/hubfs/figgro_logo_TM_Green-1.png?height=50&name=figgro_logo_TM_Green-1.png)